FDA approves not one, however two gene therapies for sickle cell illness

Gene remedy is now obtainable for sufferers with sickle cell illness. The FDA on Friday permitted remedies from Vertex Prescription drugs and Bluebird Bio, every providing a probably healing therapy for the inherited blood dysfunction.
Approval for Vertex's Casgevy got here proper on the goal date for an FDA determination and three weeks after regulators in the UK made the therapy the world's first permitted CRISPR-based remedy. For Bluebird's therapy, referred to as Lyfgenia, approval comes nearly two weeks early. The FDA's selections for each therapies apply to sufferers 12 years of age and older.
“Sickle cell illness is a uncommon, debilitating and life-threatening blood dysfunction with a big unmet want, and we’re excited to additional advance this subject, particularly for people whose lives have been severely disrupted by the illness by in the present day approving two cell-based gene therapies,” Nicole Verdun, director of the Workplace of Therapeutic Merchandise throughout the FDA's Heart for Biologics Analysis and Analysis, stated within the company's approval announcement. “Gene remedy holds the promise of delivering extra focused and efficient remedies, particularly for people with uncommon illnesses for which present therapy choices are restricted.”
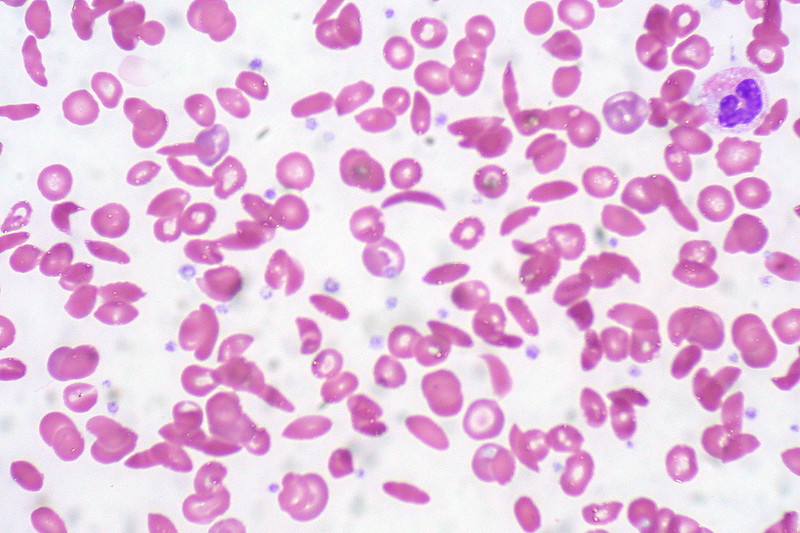
Sickle cell illness is attributable to mutations within the oxygen-carrying protein hemoglobin. Wholesome crimson blood cells are spherical and versatile. Sufferers with sickle cell illness develop crimson blood cells which are stiff and formed like crescents or sickles. These sickle-shaped cells block blood circulation, depriving the tissue of oxygen in what is named a vaso-occlusive disaster. This complication may cause painful episodes that require hospitalization. Recurrence of this complication can result in incapacity and untimely dying. A bone marrow transplant is a therapy possibility, however this process requires a matched donor and carries the chance of rejection.
Each Casgevy and Lyfgenia are made by harvesting stem cells from the affected person's bone marrow. These cells are then modified within the laboratory. Boston-based Vertex's remedy makes use of CRISPR to edit a gene in cells to provide excessive ranges of fetal hemoglobin. Primarily based in Somerville, Massachusetts, Bluebird's remedy makes use of an engineered lentivirus to switch cells to provide a model of hemoglobin that features equally to hemoglobin produced by adults who shouldn’t have sickle cell illness. Each therapies are administered to sufferers as one-time infusions.
The approvals of Casgevy and Lyfgenia gene therapies are primarily based on scientific trial knowledge exhibiting a discount in vaso-occlusive crises. The principle aim of Vertex remedy was to be freed from extreme episodes for at the least 12 consecutive months over a 24-month follow-up interval. The outcomes confirmed that 93.5% of the 31 evaluable sufferers met this determine. For Lyfgenia's pivotal research, the first goal was to measure decision of vaso-occlusive crises between six and 18 months after infusion of the remedy. The research outcomes confirmed that 88% of the 32 sufferers handled achieved this aim.
In contrast to Vertex's Casgevy, Lyfgenia's label features a black-box warning stating that instances of blood most cancers have occurred in sufferers handled with this Bluebird remedy. The label warns medical doctors to observe sufferers for indicators of most cancers. Final week, the FDA introduced that it’s investigating instances of most cancers that adopted therapy with most cancers cell therapies. Such secondary cancers are identified dangers of cell and gene therapies that use a viral vector to introduce genetic materials. The FDA stated sufferers who obtain each sickle cell gene therapies can be adopted in long-term research to additional consider security and efficacy.
Vertex set a wholesale value of $2.2 million for Casgevy. Bluebird's Lyfgenia is dearer at $3.1 million, which the corporate says acknowledges the worth the remedy can present over time by decreasing and even eliminating the intense issues that sickle cell sufferers expertise. Bluebird will supply payers the chance to decide on outcome-based agreements that tie drug reimbursement to the achievement of scientific profit, measured over three years. Bluebird has expertise with these agreements and has carried out the same settlement for Zynteglo, a gene remedy permitted final yr for the therapy of one other uncommon blood illness referred to as beta-thalassemia.
In a notice despatched to buyers on Friday, William Blair analyst Sami Corwin stated Lyfgenia's black field warning and better value pose commercialization points for the Bluebird remedy. Leerink Companions analyst Mani Faroohar wrote in a analysis notice that each approvals are in step with expectations, however Bluebird is additional deprived by not receiving a precedence evaluation voucher, a voucher that enables for expedited FDA evaluation of one other product for uncommon illnesses attainable. Corporations can obtain these vouchers for growing new remedies for uncommon illnesses, and they’re usually bought at costs exceeding $100 million. With no voucher to promote, Bluebird gained't be capable to successfully commercialize the brand new product, Foroohar stated.
Vertex can also be looking for FDA approval for Casgevy in beta-thalassemia. A regulatory determination on that indication is predicted in March. William Blair analysts stated Casgevy's approval in sickle cell illness makes an approval for beta-thalassemia extra doubtless.
Photograph by Flickr person Ed Uthman by way of a Inventive Commons license