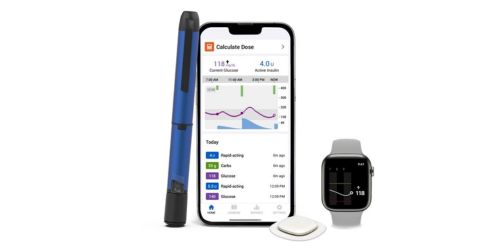
November 22, 2024 – Medtronic plc introduced US Meals and Drug Administration (FDA) approval for its new InPen™ app with missed meal dose detection, paving the best way for the launch of its Sensible MDI system with the Simplera™ steady glucose monitor (CGM) . The corporate's Sensible MDI system combines the InPen™ sensible insulin pen with the most recent Simplera™ CGM – the corporate's first disposable, all-in-one CGM that’s half the dimensions of earlier Medtronic CGMs.
With this approval, the system would be the first out there to advocate corrections for missed or inaccurate insulin doses, offering real-time, personalised insights for people receiving a number of each day injection (MDI) remedy.
For individuals with diabetes who require each day insulin injections, administering a bolus earlier than meals is important because it helps regulate glucose ranges and prevents blood sugar spikes after consuming. Minimizing the frequency of those glucose spikes reduces the danger of each short- and long-term problems and helps higher general well being. Nevertheless, it’s estimated that folks with diabetes recurrently miss 1 in 3 doses. The Missed Dose alert function helps reduce the frequency of those glucose spikes. The Medtronic Sensible MDI System takes the guesswork out of diabetes administration, serving to to handle a big unmet want for MDI customers who wrestle with making numerous insulin dosing selections day-after-day.
Proceed studying