Security stands out in Arcellx Cell Remedy's ASH information, however don't neglect the manufacturing advantages
Cell remedy developer Arcellx claims its lead program may very well be a safer different to at the moment out there CAR T therapies for a number of myeloma, noting that beforehand reported preliminary information present no worrisome neurological problems. Extra detailed information offered on the American Society of Hematology's annual assembly builds on the security profile of Gilead Sciences' collaboration Arcellx remedy, which some analysts now say has the potential to be greatest at school.
In a pivotal Part 2 trial of the Arcellx remedy, anitocabtagene autoleucel (anito-cel), 97% of sufferers responded to the one-time remedy. This research evaluates anito-cel in sufferers who’ve obtained a minimum of three prior traces of remedy. With a median follow-up of 9.5 months, 62% of sufferers achieved an entire response to anito-cel. On these efficacy measures, analysts say outcomes thus far are corresponding to these of Carvykti, the BCMA-targeted cell remedy marketed by Johnson & Johnson and Legend Biotech. However Arcellx might distinguish itself with higher security.
One concern with Carvykti is the danger of parkinsonism, a motion dysfunction. Within the Carvykti medical trials, parkinsonism was reported in 3% (eight of 285) of research members. 5 of those circumstances have been categorized as grade 3 or larger. This complication occurred later, noticed weeks after administration of remedy. The opposite out there BCMA-targeted cell remedy is Abecma, from Bristol Myers Squibb and 2Seventy Bio.
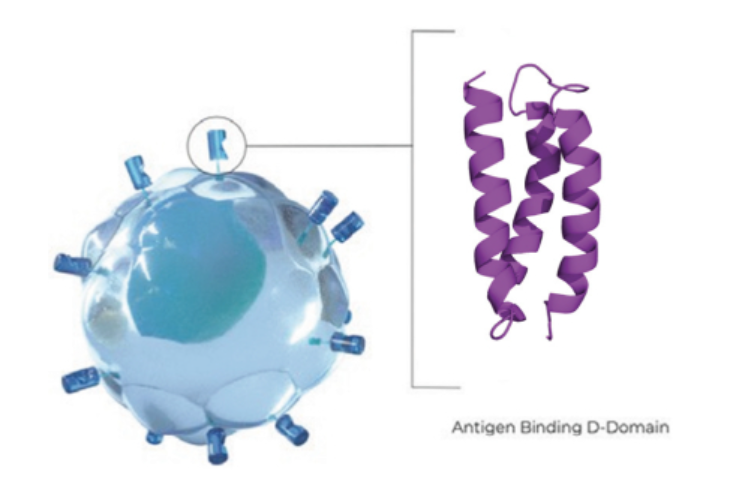
Within the greater than 150 sufferers who obtained anito-cel, Arcellx stated there have been no circumstances of parkinsonism thus far, nor have there been studies of cranial nerve palsies or Guillain-Barré syndrome, a situation through which the immune system damages nerve cells. For anito-cel, higher security lies within the design of the remedy. Autologous cell therapies are made by harvesting a affected person's immune cells and engineering them to go after a goal. Arcellx develops its therapies with an artificial binding area it calls D-Area. D area permits excessive expression of the chimeric antigen receptor for the goal protein, which within the case of anito-cel is BCMA. However this binder is designed to cut back the danger of inflicting an immune response.
Higher safety was one of many options that attracted Gilead's curiosity. Two years in the past, Gilead paid Arcellx $225 million upfront and made a $100 million fairness funding within the biotech, beginning an anito-cel alliance (identified in earlier levels of improvement as CART-ddBCMA). Relying on this system's progress, Arcellx might earn as much as $3.9 billion in milestone funds. If anito-cel is accredited, the 2 firms will share within the commercialization of the remedy.
For William Blair analyst Sami Corwin, the dearth of any delayed neurological occasions in medical testing to date is a key level of differentiation for the Arcellx remedy. In a letter to traders, she stated that whereas there have been excessive charges of an extreme immune response, referred to as cytokine launch syndrome, this complication is constant throughout the category of CAR T therapies and was manageable.
One hurdle for cell remedy is manufacturing, which takes place in a multi-step course of that may take a month or extra. Arcellx says it could possibly supply a 17-day lead time for anito-cel, leveraging the manufacturing infrastructure Kite has developed for its commercialized cell therapies. A panel of physicians talking at an Arcellx investor occasion Monday night famous that the speedy turnaround improves the general expertise of CAR T remedy for sufferers and physicians.
The method of turning a affected person's immune cells right into a residing drug is just not at all times profitable, however Arcellx stated anito-cel was efficiently manufactured for 99% of sufferers in its research. Kite, which produces the FDA-approved cell therapies Yescarta and Tecartus, claims a 96% industrial success charge in manufacturing, treating greater than 25,000 sufferers with therapies produced from a CAR T infrastructure spanning greater than 500 approved remedy facilities worldwide, together with 150 facilities within the US Corwin famous that the smaller dimension of the D area means it may be extra simply expressed by the engineered cells, offering constant ought to allow manufacturing of the tip product on a industrial scale.
“We consider Kite's popularity as a CAR T chief and established community [authorized treatment centers] will lend itself to a powerful industrial launch of anito-cel in 2026 if accredited,” Corwin stated.
Like Corwin, Leerink Companions analyst Daina Graybosch believes anito-cel has best-in-class potential, each when it comes to security and the manufacturing advantages that Gilead's Kite division brings. In a analysis be aware, she described Kite as “head and shoulders above the sphere in relation to cell remedy manufacturing and operational excellence.” Nevertheless, Arcellx manufacturing is at the moment performed by contractors Lonza and Oxford Biomedica. Graybosch stated there’s some uncertainty as as to if Kite can full the know-how switch for the remedy in a well timed method and whether or not the pace and high quality of manufacturing will translate to anito-cel, particularly for the reason that Arcellx remedy makes use of a lentivirus for supply, whereas Kite's present therapies use a gamma retrovirus.
Arcellx is at the moment enrolling sufferers for a part 3 take a look at with anito-cel. This research will consider cell remedy in sufferers who’ve obtained one to 3 prior traces of remedy for a number of myeloma. The Part 3 research will leverage Kite's manufacturing capabilities, Arcellx stated in an investor presentation.
Illustration from Arcellx's IPO prospectus