That’s the subtitle of a paper by Vreman et al. (2020). The authors have a look at all remedies authorised by FDA and EMA that had been subsequently assessed by HTA authorities in each jurisdictions between 1995 and 2018. The authors used Icer because the US HTA company; European HTA our bodies embrace IQWIG (Germany), Good (VK), ZIN (the Netherlands) and EUNETHTA. From these studies, the authors labeled any uncertainties talked about in six classes:
- Security: Small pattern dimension, causality of negative effects uninterpretable, lengthy -term security
- Take a look at validity: choice bias, efficiency bias, detection bias, course bias, reporting bias
- Inhabitants: Inhabitants doesn’t correspond to follow, subgroups that haven’t been sufficiently studied/reported
- Intervention: Unreliable or lacking details about interactions with different medicine, unreliable or lacking details about monotherapy or mixture regime, unreliable or lacking details about the right length of therapy
- Comparative results: Unreliable or lacking details about results towards related comparators, unreliable oblique comparisons, unreliable or lacking details about the right therapy line
- Outcomes: Unreliable or lacking details about lengthy -term results, related outcomes that aren’t measured or reported
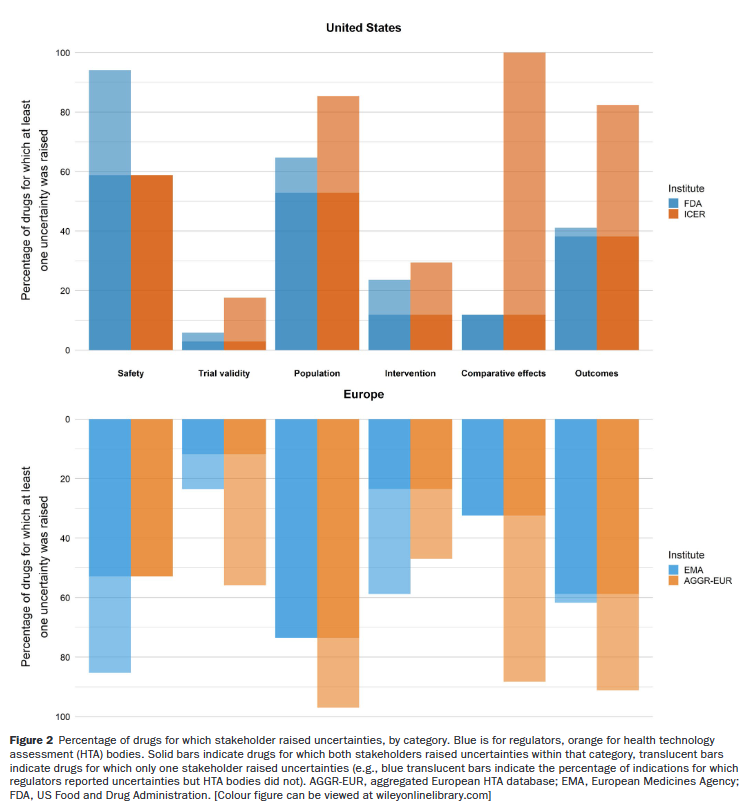
With the assistance of this method, the authors evaluated 33 medicines, which cowl 34 indications. The authors found that 7.4 uncertainties (SD 3.8) per medication per establishment had been elevated, whereby HTA authorities would in all probability improve uncertainties in comparison with supervisors. What did the authors assume:
Security issues – equivalent to these associated to pattern dimension or uncertainties in causality – had been elevated by supervisors for nearly all medicines rated (94% for the FDA and 85% for the EMA). HTA authorities introduced security issues for under 59% (ICER) and 53% (AGGR-EUR) of medicines …
HTA authorities elevated uncertainties with regard to results towards related comparators for nearly all medicines (100% in america and 88% in Europe), whereas this class was hardly tackled by the FDA (12%) and solely barely extra by the EMA (32 (32 %).
One can visually see the breakdown per class within the graph under.

Fascinating all over the place. You possibly can learn the total paper right here.