The impact of suspending the choice of medicine for small molecules for the negotiation of Medicare Drug Worth
President Trump has simply signed an govt order with varied proposals on prescription drug costs, together with efforts to “enhance” the inflation discount regulation, signed by President Biden in 2022 with varied provisions to decrease the drug prices for folks with Medicare and scale back drug spending by the federal authorities. Within the new govt order, the HHS secretary is instructed to work with the congress to carry out a change within the Medicare Drug Worth Negotiation Program to postpone the negotiation of so -called “small molecule” medicines than 7 years after the FDA approval below present laws. This transformation would imply that medicine for small molecules can be available on the market for longer earlier than they’re eligible to be chosen for Medicare Drug Worth negotiations, which may result in greater Medicare outputs for prescribed drugs, greater costs and probably greater Medicare half D premies.
Based on present laws, high-quality medicines could be chosen for negotiations if they’re model medicines or natural merchandise with out generic or biosimilar equivalents, and not less than 7 years (for medicine with small molecule) or 11 years (for organic companies) past their FDA approval or licensed date when the cms). This interprets in 9 years for medicines for small molecules or 13 years for biology after approval of the FDA when the negotiated costs of Medicare come into impact. In accordance with the Government Order of the brand new Trump administration, some members of the Congress proposed laws supported by the pharmaceutical business to launch medicine for small molecules of choice for one more 4 years, in order that each kinds of medicines can be 11 years available on the market to be eligible for choice.
In comparison with organic medicines, medicine for small molecules, which regularly tackle the form of drugs or tablets, often cheaper and simpler to producer, take it simpler and on common cheaper. Consequently, the shorter time-frame for the choice of medicines for small molecules by its critics is characterised as a so -called “capsule penalty”, whereby the pharmaceutical business claims that the eligible for medicine with small molecule is eligible for negotiations in these medicines. Nonetheless, altering the regulation to additional postpone the choice of medicine for small molecule for Medicare value negotiations would yield prices for medication and beneficiaries by giving pharmaceutical firms 4 additional years to find out their very own costs for these medicine earlier than they’re eligible for negotiation by the federal authorities, except mixed with greater spending.
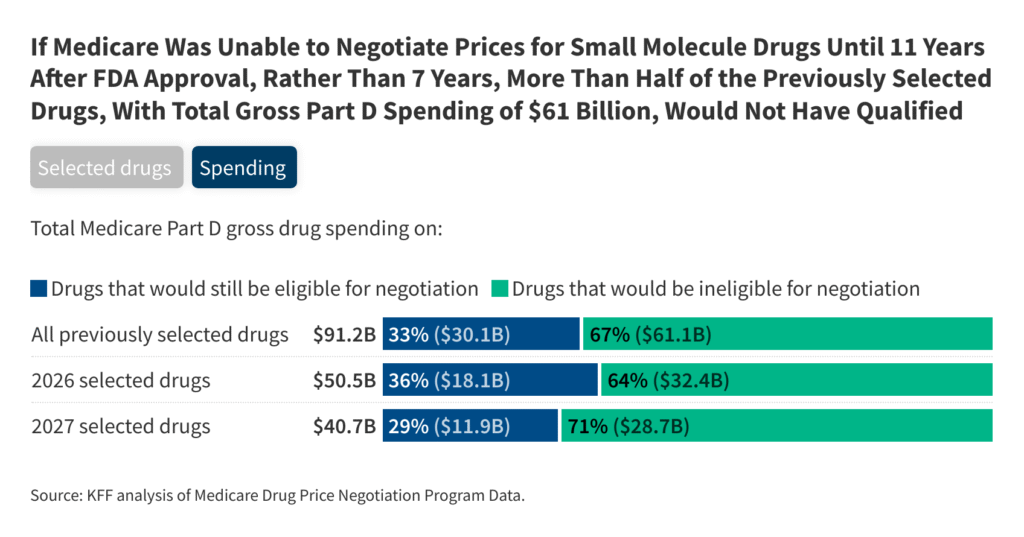
If Medicare have been to be negotiated solely 11 years after the FDA approval of the costs for medicine for small molecuuls, greater than half of the half D medicines chosen for value negotiations within the first or second rounds – 13 of the 25 – wouldn’t be thought-about when medicines have been chosen that medicines have been chosen. Through the first spherical of negotiations (for negotiated costs that may come into impact in 2026), 5 out of 10 chosen half D medicines wouldn’t have been eligible for negotiations, based mostly on the variety of years since they have been accepted by the FDA. For the second negotiation spherical (for negotiated costs in 2027), 8 out of 15 medicines wouldn’t have been chosen (Determine 1, the 'chosen medicines' tab and Desk 1).
A 4-year delay in choosing medicines for small molecules for value negotiations would have exempt varied medicines with excessive complete gross medication half D-expenditure within the first and second negotiation rounds. Eliquis and Jardiance, for instance, 2 of the highest 3 medicines based mostly on complete gross medicare half D expenditure chosen within the first spherical, wouldn’t be eligible that yr based mostly on their FDA items inspection knowledge. Likewise, 2 of the highest 3 medicines chosen within the second spherical, Ozempic/Rybelsus/Wegovy (Semaglutide) and Trelgy Ellipta, wouldn’t be eligible for choice based mostly on their approval knowledge. (Though he has an injectable type corresponding to many organic medicines, Ozempic has a molecular construction with which it may be regulated and accepted below the identical route as medicines for small molecules.)
As an instance the implications of this potential change, in accordance with present laws, small molecular medicines certified for choice in spherical two of negotiations if they’ve been accepted by the FDA not less than 7 years earlier than the publication date of February 1, 2025, earlier than February 1, or February 1, or 1 February. 1, 2025, and was eligible for choice in spherical two below present laws. Nonetheless, if the choice of medicines for small molecules was postponed for one more 4 years, as proposed, Ozempic wouldn’t be eligible for choice. By extending the interval from 7 years to 11 years after the FDA approval earlier than medicine could be chosen for small molecules for negotiation, Ozempic would solely be eligible for negotiations after 5 December 2028 and would have had 13 years after approval of the FDA earlier than the negotiated value of Medicare was taken into operation.
The 13 medicines that may not be eligible to be chosen for negotiations in the course of the first and second rounds below a delay of 4 years for medicine with small molecules have been good for two-thirds of the full gross medicare half D expenditure on the 25 chosen medicine, or $ 61 billion of $ 91 billion. The 5 small molecular medicines that may not be eligible for choice in the course of the first negotiation spherical are good for $ 32.4 billion (64%) of the full gross expenditure of $ 50.5 billion in all 10 chosen medicine. That is based mostly on expenditure between June 2022 and Could 2023, the interval used to find out the gross half D expenditure to pick medicines for the primary spherical of value negotiation (Determine 1, 'expenditure' tab).
The 8 medicines that may not be eligible for choice within the second spherical account for $ 28.7 billion (71%) of the $ 40.7 billion in complete gross half D expenditure on all 15 chosen medicines. That is based mostly on expenditure between November 2023 and October 2024, the interval used to find out the gross half D expenditure to pick medicine for the second spherical of value negotiations.
If small molecular medicines have been topic to an extra 4-year delay from their FDA approval date earlier than they’re eligible for choice within the first two rounds of Medicare-Drugs Worth Negotiations, Medicare ought to choose varied different medicines with decrease complete gross half D expenditure to finish the checklist of chosen medicines in annually. This implies that figuring out this authorized change may enhance the Medicare expenditure in comparison with present laws on account of decrease financial savings in reference to negotiations on drug value, with probably greater costs for medicines and premiums for half D registrations. Though the chief order of the Trump administration means that different reforms could be applied to stop a rise within the complete prices for medication and beneficiaries associated to this coverage change, it didn’t specify the main points of these modifications.
This work was partially supported by Arnold Ventures. KFF maintains full editorial management over all its coverage evaluation, polling and journalism actions.