Blockbuster Sanofi & Regeneron Drug Dupixent notches FDA nod for inflammatory pores and skin illness
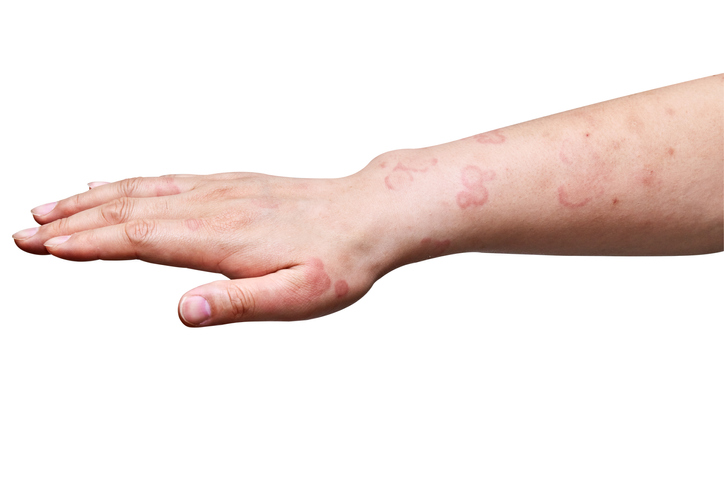
Sanofi and Regeneron Prescribed drugs Drug Dupixent has gained the FDA approval for persistent spontaneous urticaria, which introduces a brand new method to the therapy of this inflammatory pores and skin dysfunction. It’s the seventh FDA -approved indication for the blockbuster product.
The regulatory determination introduced on Friday covers the therapy of sufferers aged 12 years and older, whose persistent spontaneous urticaria (CSU) signs proceed regardless of the therapy with customary care antihistamines.
CSU is partially powered by sort 2 irritation, a type of overactive immune response. This response led to hives and itching; CSU is outlined as a illness that lasts greater than six weeks. Sanofi estimates that 1.7 million folks within the US are hit by CSU. Regardless of the broad availability of antihistamines, the corporate says that about half of the CSU sufferers have ailments which are insufficiently managed by these customary therapies.
Antihistamines give attention to H1 receptors, receptors on immune cells that play a task in immune response and irritation. The second-line therapy possibility for CSU is Xolair van Genentech, an bronchial asthma drug that has expanded its approval to the inflammatory pores and skin dysfunction in 2014. Xolair is an antibody designed to dam immunoglobulin e-receptors concerned in allergic reactions and immune reponsen. However different paths play a task in CSU. Dupixent, a drugs administered by injection each two weeks, is a monoclonal antibody designed to dam the sign routes IL-13 and IL-4.
The FDA approval of Dupixent in CSU is predicated on the outcomes of two part 3 assessments that evaluated the drug as an add-on for antihistamines, in comparison with a placebo and antihistamines. Outcomes confirmed that Dupixent achieved the first and secondary objectives to scale back the severity and itching and beehorfactivity in comparison with the management arm after 24 weeks. The research of the research drug additionally confirmed an elevated threat of well-controlled illness or an entire response after 24 weeks. With security, the outcomes had been in step with the nicely -known Dupixent security profile in his permitted indications. The commonest reported uncomfortable side effects had been reactions from injection places.
Dupixent was first permitted in 2017 as a therapy for atopic dermatitis. It’s the best-selling product from Sanofi, good for greater than € 13 billion (round $ 14.7 billion) in revenue in 2024. Sanofi's inflammatory and immunology technique contains increasing using the drug to different indications. Final yr the European Medicines Fee and the FDA Dupixent permitted persistent obstructive lung illness.
“This FDA approval provides a brand new therapy choice to deal with the underlying drivers of those critical and recurring indicators and signs,” stated Alyssa Johnsen, worldwide therapeutic space, immunology and oncology growth at Sanofi, in a ready rationalization. “Dupixent has the potential to enhance the outcomes for CSU sufferers who beforehand had restricted therapy choices.”
In 2023 the FDA rejected the primary software of Sanofi for the drug in CSU and requested for extra scientific information to point out effectiveness. That entry was based mostly on two part 3 research, considered one of which has not achieved the principle goal of the take a look at. The reorganization contained information from a 3rd part 3 take a look at. Dupixent is permitted for CSU in Japan, the United Arab Emirates and Brazil. The drug remains to be being assessed on this indication in Europe and different markets all over the world.
There are different firms that attempt to carry new approaches for the therapy of CSU. Celldex Therapeutics is scientific growth with Barzovolimabab at a late stage, an antibody drugs that’s designed to bind to the equipment receptor on manure cells. Evommune needs to deal with mast cell activation with an oral small molecule, EVO756. When the startup revealed a sequence of C finance of $ 115 million C sequence final October, it stated that the provisional part 2B information anticipated in CSU within the first half of 2025.
However CSU Drug Analysis has additionally led to failures. In 2022, the third harmonic ignited growth of an oral equipment trimmer with small molecule after part 1 information confirmed indicators of potential liver toxicity. The corporate centered on one other kit-blocking small molecule, Thb335, which is at present being ready for part 2 growth. However final week Third Harmonic introduced plans to dissolve the corporate and promote its belongings, together with Thb335, who return money to shareholders.
Within the meantime, Allakos has twice a scarcity in his efforts to develop antibodies that inhibit manure cells. Final yr Allakos reported that his drug Lirentelimab didn’t beat a placebo in a part 2 take a look at in CSU. The biotech centered on one other drugs, AK006. However the part 1 failure of that drugs induced the second industrial restructuring of the biotech up to now yr in January.
Picture: Getty photographs