Ascletis reviews optimistic ends in the Part III denifanstat research for pimples
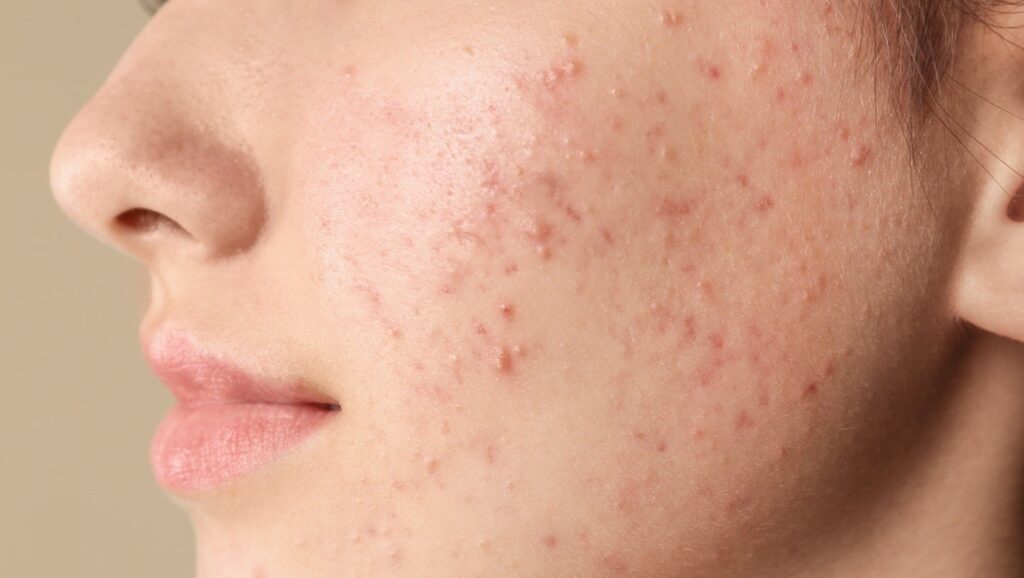
Ascletis Pharma has introduced optimistic outcomes from its Part III open-label research assessing the long-term security of denifanstat (ASC40) in sufferers with average to extreme pimples vulgaris.
The multi-centre research befell in China and concerned 240 sufferers, all of whom beforehand obtained denifanstat or placebo for 12 weeks, earlier than receiving denifanstat as soon as a day for 40 weeks.

Uncover B2B advertising that delivers
Mix enterprise intelligence and editorial excellence to achieve engaged professionals throughout 36 main media platforms.
Extra data
The first endpoints had been the incidence of treatment-emergent opposed occasions (TEAEs), severe opposed occasions (SAEs), and discontinuations as a consequence of opposed occasions (AEs).
Denifanstat demonstrated tolerability and a positive security profile, and most TEAEs had been delicate (grade one) or average (grade two). The corporate reported no grade 3 or 4 denifanstat-related opposed occasions, no associated SAEs, and no deaths through the research interval.
In June 2025, denifanstat met all main, key secondary and secondary endpoints in its earlier double-blind, placebo-controlled, randomized part III research in 480 sufferers, additionally for the therapy of average to extreme pimples vulgaris.
Denifanstat acts through twin mechanisms for the therapy of pimples: inhibition of de novo lipogenesis (DNL) in human sebocytes to immediately cut back sebum manufacturing, and inhibition of inflammatory responses by lowering cytokine secretion and Th17 differentiation.
Ascletis holds unique rights to denifanstat, a once-daily oral fatty acid synthase (FASN) inhibitor, in Larger China via a licensing settlement with Sagimet Biosciences.
Just lately, the China Nationwide Medical Merchandise Administration accepted the brand new drug utility for denifanstat for pimples.
In June 2025, Sagimet Biosciences’ denifanstat succeeded in a Part III trial. The Chinese language research, carried out by Ascletis, discovered that denifanstat achieved a therapy success of 33.2% in sufferers with pimples vulgaris who took the oral remedy for 12 weeks.
