NG Biotech Pronounces FDA Approval for Diagnostic Testing
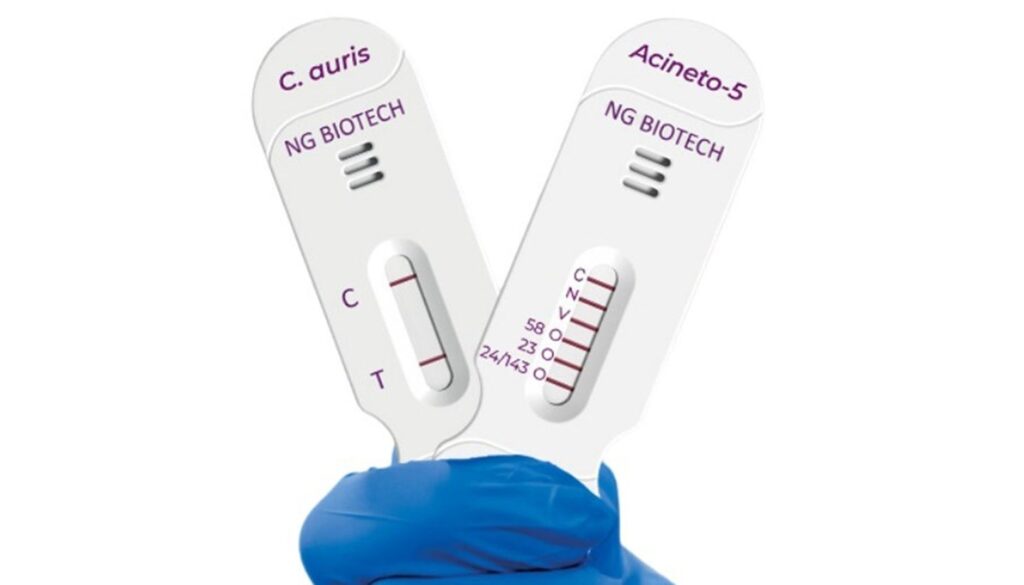
NG Biotech and Hardy Diagnostics have acquired breakthrough system designation from the U.S. Meals and Drug Administration (FDA) for 2 speedy diagnostic checks, NG-TEST Candida auris and NG-TEST Acineto-5, concentrating on vital drug-resistant pathogens.
The designation applies to pathogen testing particularly concentrating on Candida auris and Acinetobacter baumannii, which have been recognized by the World Well being Group (WHO) as international well being priorities.

Uncover B2B advertising that delivers
Mix enterprise intelligence and editorial excellence to achieve engaged professionals throughout 36 main media platforms.
Extra info
Listed within the WHO’s listing of fungal pathogens, Candida auris is a multidrug-resistant yeast answerable for hospital outbreaks with difficult detection and excessive mortality charges.
NG-TEST Candida auris is a speedy lateral movement immunoassay designed to determine the yeast in cultured samples in quarter-hour.
Included within the listing of bacterial precedence pathogens, carbapenem-resistant Acinetobacter baumannii (CRAB) might be quickly transmitted inside healthcare settings.
Printed research reveal 100% settlement with reference strategies for a number of isolates, supporting its usefulness in outbreak response and an infection management efforts.
NG-TEST Acineto-5 detects and distinguishes 5 main carbapenemase households, oxacillinase-24 (OXA-24)/143-like, OXA-23-like, Verona integron-encoded metallo-beta-lactamase (VIM), OXA-58-like and New Delhi metallo-beta-lactamase (NDM), from Acinetobacter samples and gives outcomes inside quarter-hour minutes.
This take a look at works with out polymerase chain response or specialised gear, streamlining laboratory workflows.
NG Biotech developed and produced the checks in France, with Hardy Diagnostics serving as unique distributor within the US. Each checks can be found for analysis functions solely, pending ongoing FDA evaluate.
Milovan Stankov-Pugès, CEO of NG Biotech, mentioned: “These groundbreaking designations validate each the expertise behind our checks and the actual want they handle.”
Andre Hsiung, Chief Scientific Officer of Hardy Diagnostics, mentioned: “The appointment underlines the rising urgency across the speedy detection of multidrug-resistant organisms that pose critical dangers to healthcare.”
