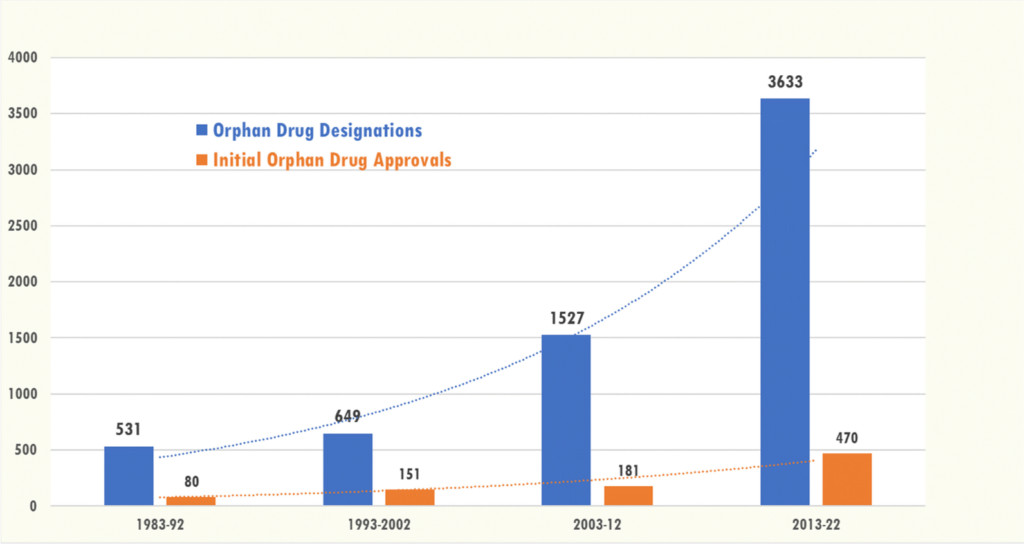
A research by Fermaglich and Miller (2023) evaluated tendencies in orphan drug designation and approval following the passage of the Orphan Drug Act of 1983 within the US. The authors use FDA information to judge between 1983 and 2022 and conclude that:
In the course of the forty years of the ODA's existence, 6,340 orphan medicine have been awarded, which equates to the event of medicine for 1,079 uncommon ailments. Moreover, 882 of those designations resulted in at the very least one FDA approval to be used in 392 uncommon ailments. A lot of this growth has been concentrated in oncology, as seven of the ten most designated and accepted ailments had been uncommon cancers.

Of the orphan drug designations (approvals), the highest 5 most necessary illness areas had been:
- Oncology: 38% of designations (38% of approvals)
- Neurology: 14% (10%)
- Infectious illness: 7% (10%)
- Metabolism 6% (7%)
- Hematology: 5% (8%)
Extra particulars on the designation and approval of orphan medicine could be discovered within the full article right here.