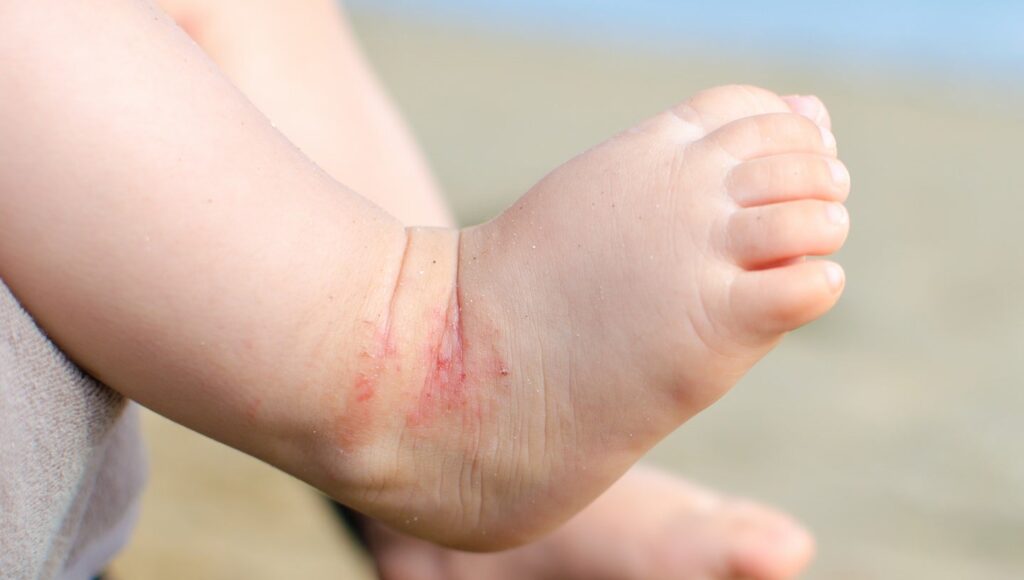
Arcutis studies constructive topline information within the Zoryve examine for pediatric AD
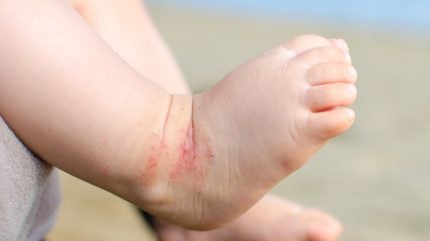
Arcutis has reported constructive topline information from the INTEGUMENT-INFANT Section II trial evaluating Zoryve (roflumilast) cream 0.05% in infants aged three months to lower than 24 months with gentle to average atopic dermatitis (AD).
Zoryve cream is a potent topical phosphodiesterase 4 (PDE4) inhibitor.

Uncover B2B advertising that delivers
Mix enterprise intelligence and editorial excellence to succeed in engaged professionals throughout 36 main media platforms.
Extra data
The multicenter, open-label INTEGUMENT-INFANT section II examine evaluated the once-daily utility of Zoryve cream 0.05% for 4 weeks in 101 infants.
This examine builds on the earlier ARQ-151-105 Maximal Utilization Pharmacokinetics (MUSE) examine, which additionally assessed Zoryve on this age group.
The information demonstrated a good security and tolerability profile, in step with earlier scientific trials of Zoryve.
The incidence of opposed occasions within the examine was low, with all reported occasions rated as gentle to average.
Zoryve each improved illness severity and diminished the realm of pores and skin affected by AD, with 58% of individuals attaining an eczema space and severity index of 75 (EASI-75) by week 4 of the examine.
David Berk, vp of R&D technique and scientific improvement at Arcutis, stated: “Reaching this scientific improvement milestone for Zoryve via the INTEGUMENT-INFANT examine underscores our dedication to offering protected, efficient nonsteroidal remedy choices for even the youngest sufferers with atopic dermatitis, who at present have a big illness burden and really restricted remedy choices.”
The findings from the Section II examine help the beforehand noticed tolerability and security of Zoryve cream 0.05% as noticed throughout the four-week INTEGUMENT-PED pivotal examine in kids aged two to 5 years.
The commonest unwanted side effects, every reported in 3% or extra of the 101 individuals, included nasopharyngitis, diarrhea, vomiting, and higher respiratory tract an infection. Just one participant discontinued as a consequence of an opposed occasion, with no severe opposed occasions noticed.
In June 2025, Arcutis enrolled the primary youngster in an open-label Section II trial of Zoryve for AD.
