Medtronic receives FDA approval for Stealth AXiS spinal robotics platform
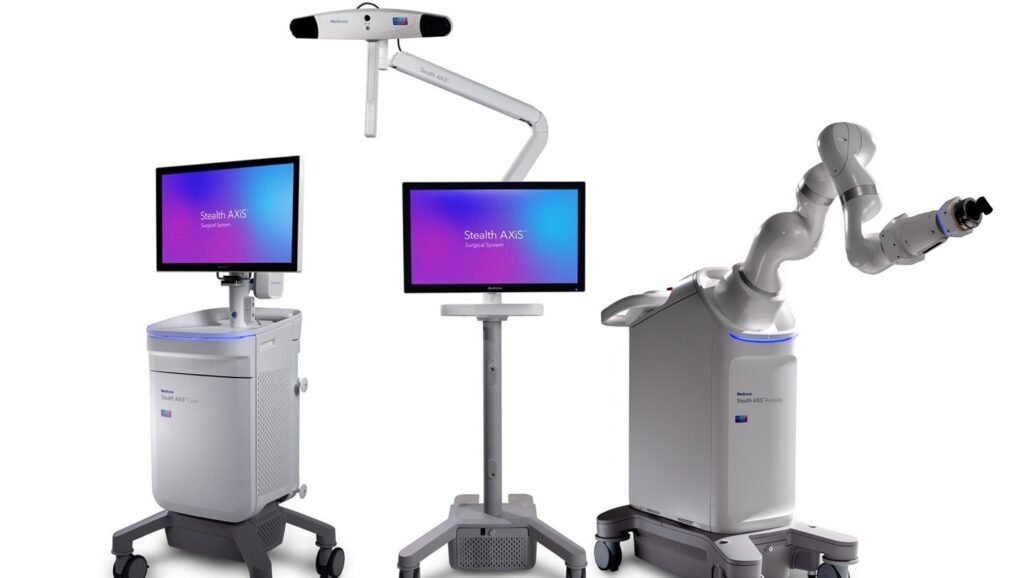
Medtronic has acquired approval from the US Meals and Drug Administration (FDA) for the Stealth AXiS robotic platform for backbone surgical procedure.
The system is designed as an built-in answer that mixes robotics, planning and navigation right into a single platform for backbone procedures within the US.

Uncover B2B advertising that delivers
Mix enterprise intelligence and editorial excellence to succeed in engaged professionals throughout 36 main media platforms.
Extra data
Medtronic goals to allow hospitals and ambulatory surgical facilities to carry out these procedures with out counting on a number of separate applied sciences.
The system’s structure permits help for quite a lot of surgeon preferences and scientific complexities.
Medtronic has constructed this platform to fulfill present wants whereas offering the chance for future growth into cranial and ear, nostril and throat (ENT) purposes, topic to additional 510(ok) clearance.
The modular design permits establishments to deploy solely what they want now and scale their capabilities as their scientific necessities change.
A key function of the Stealth AXiS system is LiveAlign segmental monitoring, which gives real-time visualization of anatomical actions, surgical changes and affected person alignment throughout backbone surgical procedure.
This performance reduces the necessity for repetitive imaging and minimizes reliance on handbook workflow steps, supporting constant execution of patient-specific surgical plans.
The Stealth AXiS system is a core a part of Medtronic’s AiBLE sensible ecosystem by integrating planning, navigation and execution right into a single workflow. This strategy streamlines surgical processes and permits seamless change of data earlier than, throughout and after surgical procedure.
The native connectivity inside the AiBLE ecosystem is designed to attach units, software program and information to allow extra significant sharing of insights throughout the surgical continuum.
Michael Carter, senior vice chairman and president of cranial and spinal applied sciences at Medtronic, stated: “Backbone surgical procedure is advanced and variability stays an actual problem.
“The Stealth AXiS system is designed to make superior know-how extra helpful and clinically significant, permitting surgeons to ship extra predictable, personalised care whereas laying the muse for continued innovation.”
Earlier this month, Medtronic reported three milestones within the US, increasing entry to its MiniMed 780G insulin supply system for people with kind 1 diabetes (T1D) and insulin-dependent kind 2 diabetes (T2D).
