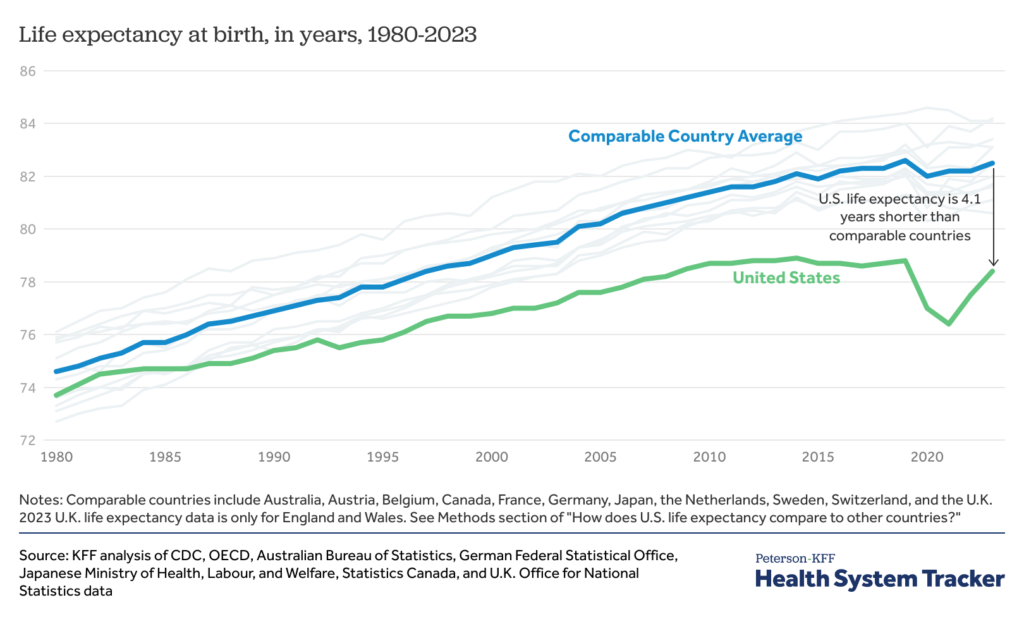
Though Medicare stays the dominant payer within the well being of house, many suppliers work to change into a 'versatile' payer. Livewell Companions is such an organization. “We outline payers' flexibility as the power to simply accept sufferers from our reference companions, no matter insurance coverage,” Jason Growe, CEO of Livewell Companions, informed Residence Well being Care Information in regards […]