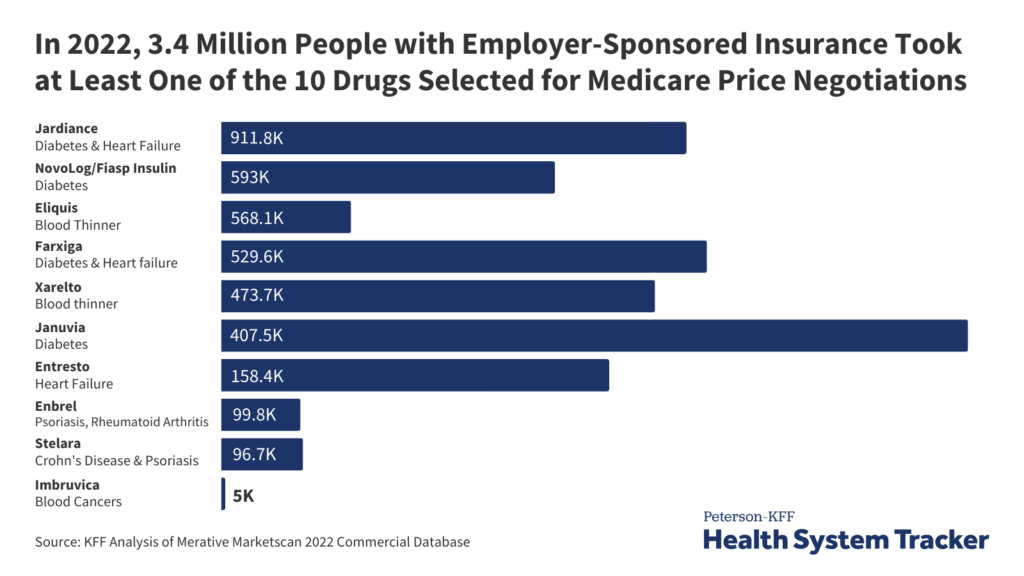
The digital commentary system makes use of the PEWS to watch youngsters's well being. Credit score: Dmytro Sheremeta / Shutterstock. Shrewsbury and Telford Hospital NHS Belief (SaTH) within the UK has launched a brand new digital paediatric commentary system, aiming to enhance look after younger sufferers. The digital improve, which replaces conventional paper data, has been rolled out throughout the […]